Équipe
Corinne BOUSQUET
MICROPANC :
Micro-environnement et Résistance Thérapeutique dans les Néoplasmes Pancréatiques
Les spécificités
de notre axe de recherche
L’adénocarcinome canalaire pancréatique (PDAC), communément appelé cancer du pancréas, est une tumeur très agressive puisqu’elle présente un potentiel métastatique rapide et une forte résistance thérapeutique. Cette tumeur présente la particularité d’être riche en stroma (jusqu’à 80% de son volume tumoral), composé d’une matrice extracellulaire très fibrotique, et de cellules stromales immunitaires et non-immunitaires, dont les cancer-associated fibroblasts (CAFs) sont majoritaires. Mon équipe de recherche s’intéresse plus spécifiquement au rôle des CAF dans l’agressivité des cellules cancéreuses pancréatiques, dans l’objectif de comprendre la biologie des CAFs, leurs dialogues avec les cellules cancéreuses et avec les autres cellules stromales (endothéliales et immunitaires), et comment ces dialogues impactent la résistance des cellules cancéreuses aux chimiothérapies ou aux immunothérapies. La compréhension de ces dialogues inter-cellulaires et avec la matrice extracellulaire nous permet de proposer et de tester des thérapies anti-cancéreuses co-ciblant les cellules cancéreuses et leur stroma, notamment par des approches de nanothérapies ciblées (projet de Véronique Gigoux).
Ainsi, pour comprendre les dialogues tumeur – stroma corrélés à l’agressivité du cancer du pancréas, notre équipe, i/ développe des modèles intégrant ce stroma et mimant la pathologie chez le patient, tels que les PDX (Patient-Derived Xenografts), et les primocultures de CAFs et de cellules tumorales cultivées en 3 dimensions (organoïdes tumoraux) et en co-culture avec les CAFs, ainsi que des modèles de cancer de pancréas murin (souris génétiquement modifiées développant un cancer spontané, et greffe chirurgicale orthotopique de cellules cancéreuses pancréatiques avec des CAFs) ; et ii/ réalise des analyses séparées de l’expression génique dans le compartiment épithélial tumoral et dans le compartiment stromal, par des approches de transcriptomique (expression des ARNm), de traductomique (expression des ARN traduits, projet d’Yvan Martineau), et de matrisome (analyse exhaustive de l’expression des protéines matricielles, projet de Christine Jean) sur les PDX (modèles tumoraux greffés hybrides) naïfs de traitement ou rendus chimiorésistants (projet de Corinne Bousquet), et/ou par déconvolution bioinformatique sur des bulk RNAseq de tumeurs humaines.
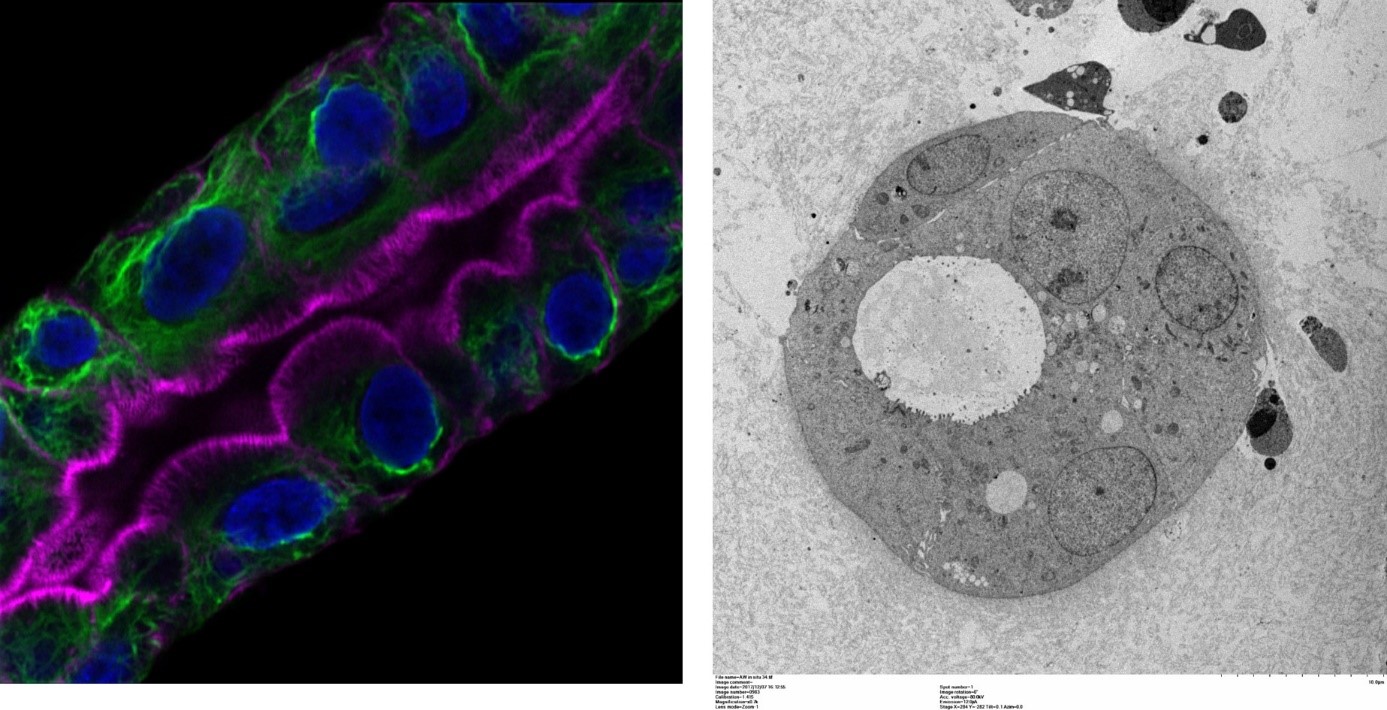
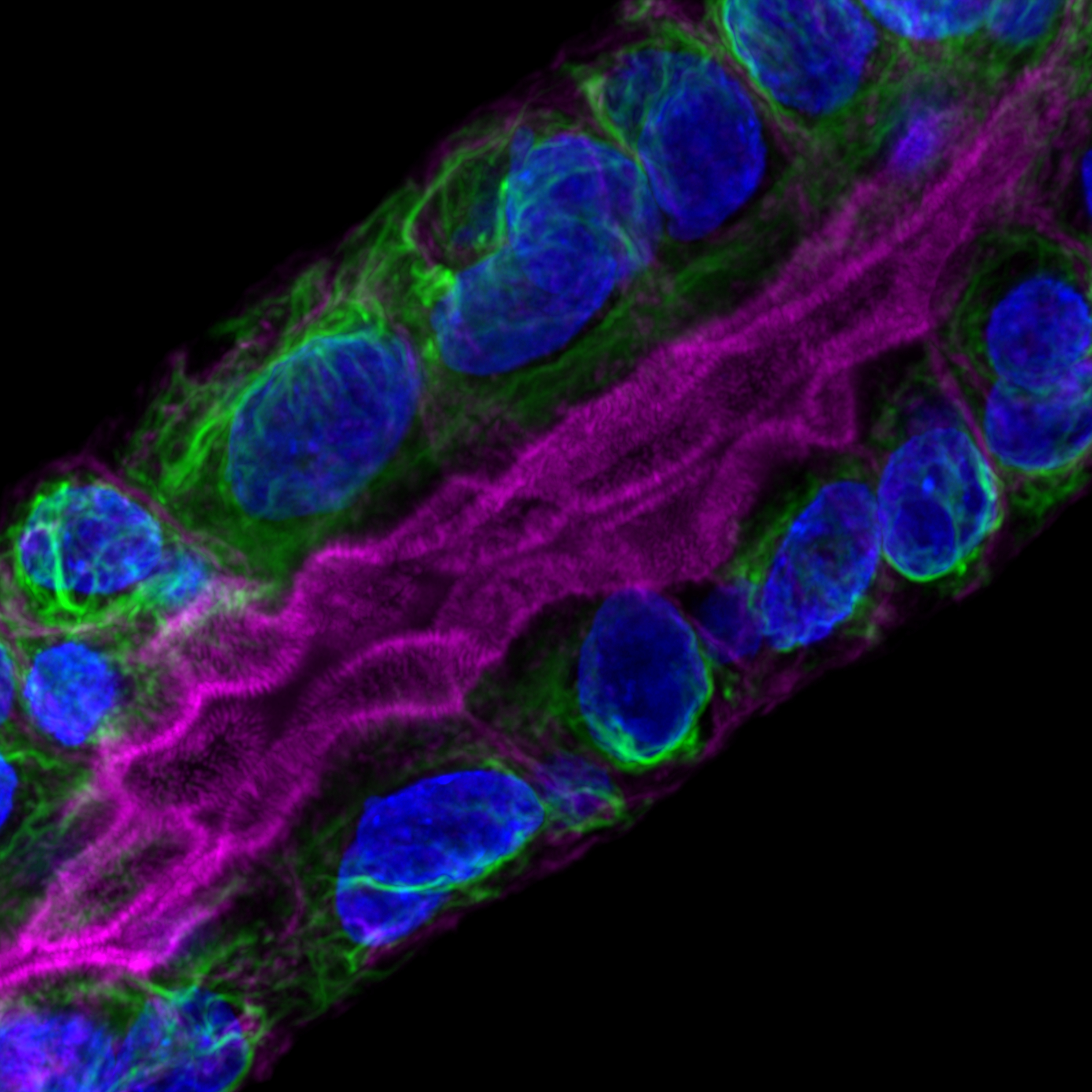
Organoïde tumoral (gauche : immunofluorescence permettant de visualiser le cytosquelette de cytokératines en vert, les filaments d’actine particulièrement abondants au niveau de la bordure en brosse en rose et les noyaux en bleu ; droite : microscopie électronique à transmission).
Cancer du pancréas
Microenvironnement & Cancer-Associated Fibroblasts
Métastases
Chimiorésistance
Régulation traductionnelle
Reprogrammation métabolique
Rigidité matricielle & mécano-transduction
Modèles expérimentaux dérivés de patients
Nanothérapies ciblées
(polysome) RNAseq
& bio-informatique
Matrisome
Imagerie tissulaire multiplexée
DES PROJETS
DE RECHERCHE
Identification des dialogues Tumeur-Stroma impliqués dans l’agressivité du cancer pancréatique
Corinne Bousquet
Nanothérapies ciblées appliquées à l’adénocarcinome pancréatique
Véronique Gigoux
Rôle de la Focal-Adhesion Kinase (FAK) fibroblastique dans l’adénocarcinome pancréatique
Christine Jean
L’analyse de la traduction des ARNm comme nouvelle approche de classification des tumeurs du pancréas
Yvan Martineau
LES FOCUS
DE L’ÉQUIPE
Découvrir
Comprendre
Participer
Aucun résultat
La page demandée est introuvable. Essayez d'affiner votre recherche ou utilisez le panneau de navigation ci-dessus pour localiser l'article.
PRODUCTIONS SCIENTIFIQUES
PUBLICATIONS 2024
PUBLICATIONS 2023
PUBLICATIONS 2022
PUBLICATIONS 2021
PUBLICATIONS 2020
PUBLICATIONS 2019
LES MEMBRES DE L’ÉQUIPE
LES PARTENAIRES & FINANCIERS

Centre de Recherches en Cancérologie de Toulouse (Oncopole)
Toulouse – FR
CRCT-Oncopole Suivre 1,220 1,117
Compte officiel du Centre de Recherches en Cancérologie de Toulouse
Castelnau-Montratier célèbre la lutte contre le cancer. Jeudi 24 août 2023 conférence avec Frédéric Chibon responsable de l'équipe @OncoSarc du @crctoncopole accompagné de Gwenaël Ferron et Martin Gauthier oncologue @IUCTOncopole
Découvrir l'équipe
➡️https://www.crct-inserm.fr/oncosarc/
Nous contacter
05 82 74 15 75
Envie de rejoindre
L’équipe du CRCT ?