Équipe
Bruno ségui – nathalie andrieu
MELASPHINX :
Métabolisme du céramide dans le mélanome : des mécanismes moléculaires à l’immunothérapie
Présentation générale de l’équipe
Notre équipe a été créée en 2011, sous la direction du Pr. Thierry Levade. Depuis 2021, elle est dirigée par le Pr. Bruno Ségui et le Dr. Nathalie Andrieu-Abadie. Elle est composée de 26 membres, incluant 3 chercheurs/6 enseignants-chercheurs, 5 ingénieurs/techniciens, 2 praticiens hospitaliers, 2 post-doctorants, 5 doctorants et 3 stagiaires de master par an.
Objectifs scientifiques
Le mélanome cutané représente la principale cause de décès parmi les cancers de la peau. Les inhibiteurs de points de contrôle immunitaire (ICI) ont révolutionné la prise en charge thérapeutique des patients atteints du mélanome cutané avancé. Toutefois, seule la moitié des patients répond de façon durable aux ICI en raison de mécanismes de résistance primaires ou adaptatifs. Des profils lipidiques anormaux sont souvent associés à un phénotype métabolique altéré dans les cellules tumorales, qui est une caractéristique du cancer. L’activité scientifique de notre équipe vise à mieux comprendre les mécanismes moléculaires par lesquels les altérations du métabolisme du céramide contribuent à la progression du mélanome, à l’échappement immunitaire et à la résistance aux ICI dans des modèles précliniques de mélanome. Grâce aux collaborations existantes entre les chercheurs et les cliniciens, notre ambition est de transférer nos découvertes scientifiques fondamentales à la clinique dans le but d’identifier de nouveaux biomarqueurs permettant de prédire la réponse aux ICI et de proposer des traitements adaptés aux patients atteints de mélanome avancé. En outre, nos travaux de recherche, réalisés au cours des 5 dernières années, ont constitué le rationnel scientifique de trois essais cliniques chez 150 patients atteints de mélanome avancé, traités par immunothérapie. Ces essais sont sous la responsabilité du Pr. Nicolas Meyer, oncodermatologue à l’IUCT.
Notre objectif ultime est de reprogrammer le métabolisme du céramide pour améliorer l’efficacité thérapeutique.
Les spécificités
de notre axe de recherche
Mélanome
Métabolisme
Céramide
Immunothérapie
TNF
DES PROJETS
DE RECHERCHE
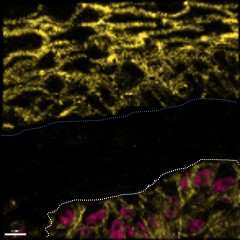
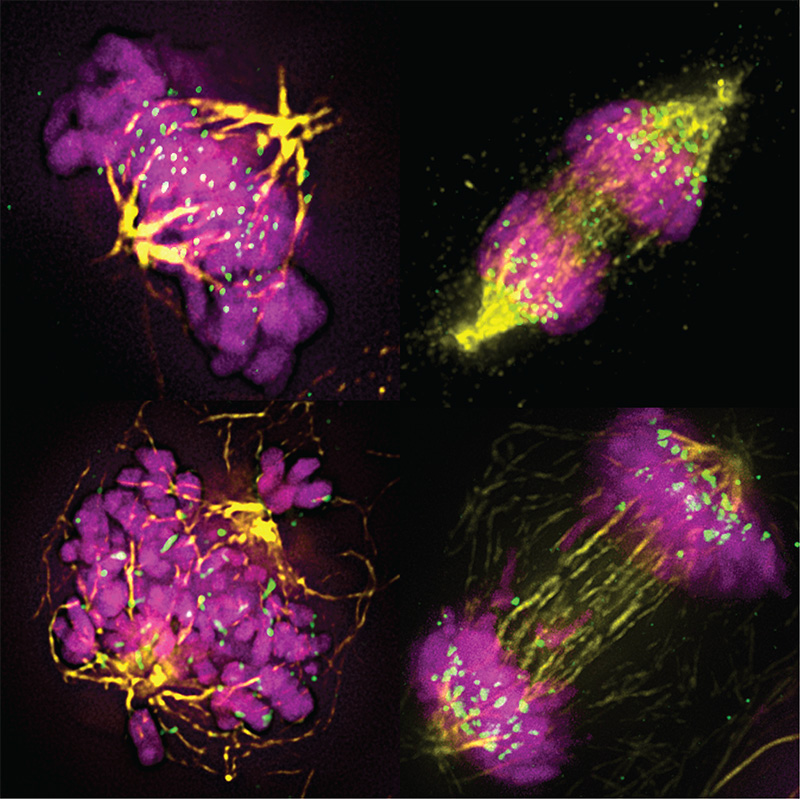
Rôle du métabolisme du céramide dans l’homéostasie épidermique et la progression du mélanome
Nathalie Andrieu
Investigateurs associés : Joëlle Riond et Frédérique Sabourdy
Interconnexions entre la signalisation du TNF et métabolisme du céramide dans le mélanome
Bruno Ségui
Investigateurs associés : Nathalie Andrieu, Céline Colacios, Anne Montfort
Reprogrammation du métabolisme du céramide pour lever la résistance aux immunothérapies
Céline Colacios, Anne Montfort
Investigateurs associés : Nathalie Andrieu, Thierry Levade, Laurence Nieto
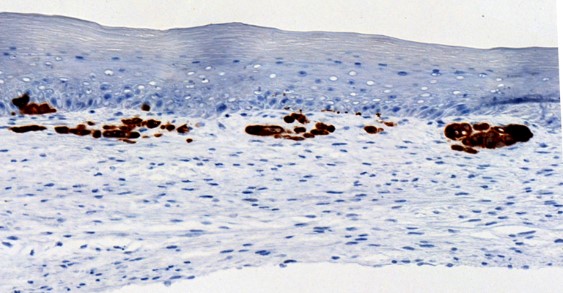
Les métabolites et les enzymes du métabolisme du céramide comme biomarqueurs de résistance aux immunothérapies ?
Nicolas Meyer
Investigateurs associés : Nathalie Andrieu, Céline Colacios, Anne Montfort et Bruno Ségui
Le TNF, une nouvelle cible en immunothérapie ?
Nicolas Meyer
Investigateurs associés : Anne Montfort et Bruno Ségui
LES FOCUS
DE L’ÉQUIPE
Découvrir
Comprendre
Participer
Aucun résultat
La page demandée est introuvable. Essayez d'affiner votre recherche ou utilisez le panneau de navigation ci-dessus pour localiser l'article.
En live
#Postdoctoral fellow at the #Cancer Research Center, Toulouse, France on the impact of combining #TNF blockade and #immunotherapy on #immune responses and #melanoma liver metastases in #mice.
2 year contract from Nov 1, 2023
Contact bruno.segui@inserm.fr before October 15, 2023.
PRODUCTIONS SCIENTIFIQUES
PUBLICATIONS 2024
PUBLICATIONS 2023
PUBLICATIONS 2022
PUBLICATIONS 2021
PUBLICATIONS 2020
PUBLICATIONS 2019
LES MEMBRES DE L’ÉQUIPE
LES PARTENAIRES & FINANCIERS

Centre de Recherches en Cancérologie de Toulouse (Oncopole)
Toulouse – FR
CRCT-Oncopole Suivre 1,220 1,117
Compte officiel du Centre de Recherches en Cancérologie de Toulouse
Castelnau-Montratier célèbre la lutte contre le cancer. Jeudi 24 août 2023 conférence avec Frédéric Chibon responsable de l'équipe @OncoSarc du @crctoncopole accompagné de Gwenaël Ferron et Martin Gauthier oncologue @IUCTOncopole
Découvrir l'équipe
➡️https://www.crct-inserm.fr/oncosarc/
Nous contacter
05 82 74 15 75
Envie de rejoindre
L’équipe du CRCT ?