The level of von Willebrand factor is a pronostic marker in patients with metastatic melanoma treated by immune checkpoint inhibitors
Immunotherapy;
Melanoma;
Translational Medical Research;
Tumor Microenvironment.
Laura Keller– Team SIGNATHER : Cell Signalling, Oncogenesis and Therapeutics
An increased incidence of thrombotic complications associated with an increased mortality rate has been observed under immune checkpoint inhibition (ICI). Recent investigations on the coagulation pathways have highlighted the direct role of key coagulatory proteins and platelets in cancer initiation, angiogenesis and progression. The aim of this study was to evaluate the prognostic value of von Willebrand factor (vWF) and its regulatory enzyme a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13), D-dimers and platelets in a cohort of patients with metastatic melanoma receiving ICI.
In this prospective cohort, coagulatory parameters such as ADAMTS13 activity and D-dimers are associated with overall survival (OS) but baseline vWF:Ag levels appeared as the only parameter associated with response and OS to ICI. This highlights a potential role of vWF as a biomarker to monitor ICI response of patients with malignant melanoma.
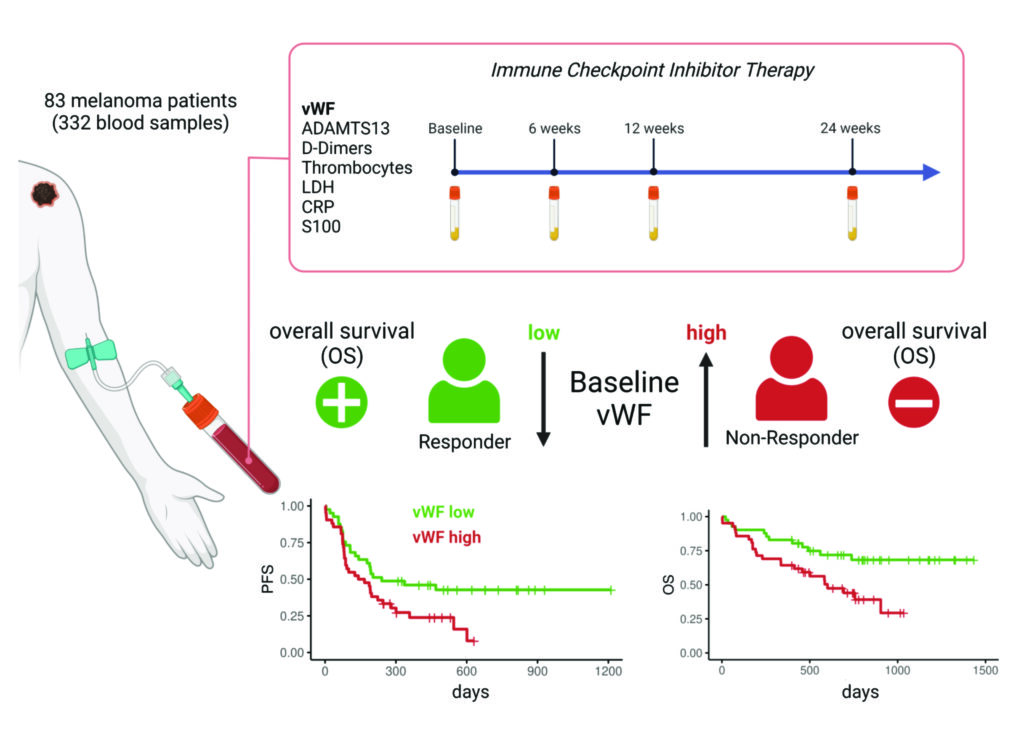
Elevated plasma levels of von Willebrand factor (vWF) are associated with a poor response to immune checkpoint therapy (ICT). Baseline vWF levels define progression-free survival (PFS) and overall survival (OS). Our results highlight vWF as a potential biomarker of response to ICI therapy and tumour progression.
Discover the published article
J Immunother Cancer. 2023 May;11(5):e006456. doi: 10.1136/jitc-2022-006456.
Prognostic value of von Willebrand factor levels in patients with metastatic melanoma treated by immune checkpoint inhibitors.
Stadler JC, Keller L, Mess C, Bauer AT, Koett J, Geidel G, Heidrich I, Vidal-Y-Sy S, Andreas A, Stramaglia C, Sementsov M, Haberstroh W, Deitert B, Hoehne IL, Reschke R, Haalck T, Pantel K, Gebhardt C, Schneider SW.
Collaborations and acknowledgements
The authors would like to warmly thank the Hiege Stiftung and the Roggenbuck Stiftung and Novartis (S2TAF-058).
LK was financially supported by ITMO Cancer ‘Plan Cancer 2014-2019’ and received funding from Fondation de France. LK and KP were financially supported by KMU- innovativ-23 n°031B0843D.
CS, JK, GG and IH received partial funding of a one- year Clinical Scientist fellowship by the Mildred
Scheel Nachwuchszentrum, UKE Hamburg (HaTriCS4). BD was financially supported by IPRime Scholarship, UKE, Hamburg.

Centre de Recherches contre le Cancer de Toulouse (Oncopole)
Toulouse - FR
Nous contacter
+33 5 82 74 15 75
Nous rejoindre ?




