
VERONIQUE GIGOUX
Targeted nanotherapies applied to
pancreatic adenocarcinoma
Certain receptors/proteins, overexpressed by tumor cells or present in the tumor microenvironment, may constitute targets for the development of new therapeutic strategies, in particular targeted nanotherapy approaches based on the use of vectorized nanoparticles. Magnetic nanoparticles offer new opportunities for tumor imaging and anti-cancer therapy.
Our knowledge of the pharmacology of the cholecystokinin type 2 receptor (CCK2R), its overexpression in certain tumors, as well as its internalization properties, has allowed us to establish proofs of concept for the development of targeted nanotherapy strategies. Magnetic iron oxide nanoparticles (MNPs) (approved for medical use as imaging contrast agents), decorated with gastrin (CCK2R specific agonist) for targeting and a fluorescent probe for detection, specifically recognize CCK2R expressing cells, internalize and accumulate in the lysosomes of targeted cells.
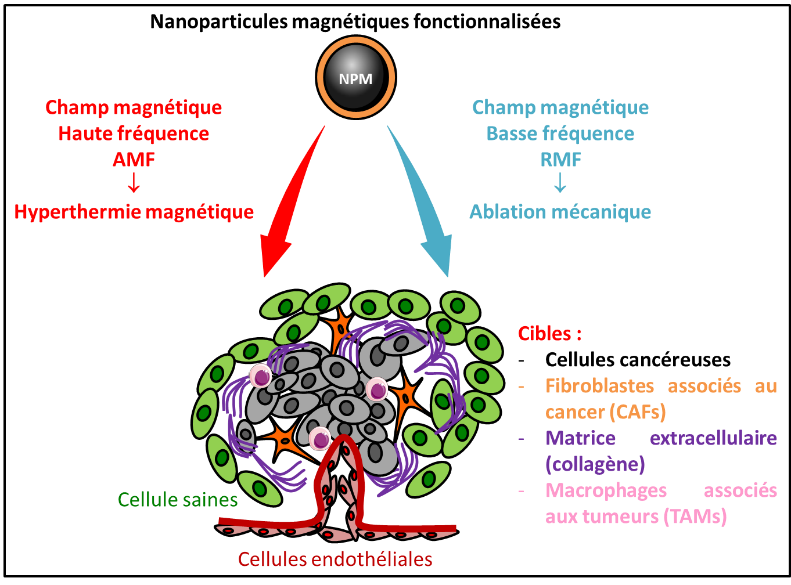
The first strategy developed is to eradicate the cells by targeted magnetic hyperthermia. The application of a high-frequency alternating magnetic field (AMF) induces the death of cancer or CAFs cells that have internalized the functionalized nanoparticles according to a lysosomal death mechanism (Sanchez 2014). Cell death is initiated in lysosomes: the mechanism involves a rise in temperature at the surface of the MNPs that generates the production of reactive oxygen species (ROS), leading to the permeabilization of the lysosomal membrane and the leakage of lysosomal enzymes, including Cathepsin B, into the cytosol. Cathepsin B then activates a Caspase-1 dependent non-apoptotic cell death pathway (Connord 2015 Hallali 2016, Clerc 2018). This targeted magnetic hyperthermia approach acts synergistically with chemotherapeutic treatment to eradicate tumor cells more efficiently, by activating two cell death pathways that are dependent on Caspase-1 and apoptotic Caspase-3, respectively (El Hajj Diab 2018).
The second strategy developed very recently consists in eradicating target cells by magneto-induced and targeted mechanical ablation. Exposed to a low-frequency rotating magnetic field (RMF), NPMs generate mechanical forces capable of inducing tumor or microenvironment cell death, and inhibiting their cell proliferation (Lopez S, 2021).
Our research is focused on the understanding of cellular (migration…) and molecular (intracellular signaling…) mechanisms induced by MNPs exposed to different magnetic fields, the optimization of these strategies with new generations of MNPs and the targeting of new tumor components (tumor-associated macrophages TAMs, extracellular matrix) (Belkahla 2020, Camacho-Fernandez 2021). In parallel, we will validate the efficacy of these strategies in pre-clinical studies in mouse models of cancer, in particular by combining them with chemotherapeutic and/or radiotherapeutic treatments.
Funding: H2020-MSCA-ITN, Plan Cancer, Agence Nationale de la Recherche, Ligue Nationale contre le cancer (thesis funding), Ligue régionale contre le cancer, Cancéropôle GSO.
Members: Véronique Gigoux (CRCN Inserm), Pascal Clerc (Study engineer Inserm), Loubna Laib (post-doctoral fellow, ANR), Justine Journaux (Thesis, LNCC), Ahmed Abdelhamid (Thesis H2020-MSCA-ITN)
References:
- Camacho-Fernandez JC, Quijano GKG, Severac C, Dague E, Gigoux V, Santoyo-Salazar J, Martinez-Rivas AMR. Nanobiomechanical behavior of Fe3O4@SiO2 and Fe3O4@SiO2-NH2 nanoparticles over HeLa cells interfaces. Nanotechnology (2021).
- Belkahla H, Antunes JC, Lalatonne Y, Sainte Catherine O, Illoul C, Journé C, Jandrot-Perrus M, Coradin T, Gigoux V, Guenin E, Motte L, Helary C. USPIO-PEG nanoparticles functionalized with a highly specific collagen-binding peptide: a step towards MRI diagnosis of fibrosis. J Mater Chem B. (2020).
- El Hajj Diab D, Clerc P, Serhan N, Fourmy D, Gigoux V. Combined treatments of magnetic intra-lysosomal hyperthermia with doxorubicin promotes synergistic anti-tumoral activity. Nanomaterials. 8:468 (2018).
- Clerc P, Jeanjean P, Halalli N, Gougeon M, Pipy B, Carrey J, Fourmy D, Gigoux V. Targeted Magnetic Intra-lysosomal Hyperthermia produces lysosomal reactive oxygen species and causes Caspase-1 dependent cell death. Controlled Release. 270: 120-134 (2018).
- Halalli N, Clerc P, Fourmy D, Gigoux V, Carrey J. Influence on cell death of high frequency motion of magnetic nanoparticles during magnetic hyperthermia experiments. Applied Physics Letters 109 (3): 10.1063 (2016).
- Connord V, Clerc P, Hallali N, El Hajj Diab D, Fourmy D, Gigoux V*, Carrey J*. Real-time Analysis of Magnetic Hyperthermia Experiments on Living Cells under Confocal Microscope. Small 11(20):2437-45 (2015).
- Sanchez C, El Hajj Diab D, Connord V, Clerc P, Meunier E, Pipy B, Payré B, Tan RP, Gougeon M, Carrey J, Gigoux V, Fourmy D. Targeting a G-Protein-Coupled Receptor Overexpressed in Endocrine Tumors by Magnetic Nanoparticles To Induce Cell Death. ACS Nano 8(2):1350-63 (2014).

