
Role of metabolism in radiation-induced plasticity and heterogeneity of Glioma stem cells
Anthony Lemarié
This translational research team, in the context of its work on the radiosensitisation of glioblastomas, has set up a new research axis on the involvement of glioblastoma stem cells in the radioresistance of these tumours. In 2010, this group initiated a project focusing on the plasticity of Glioblastoma cells in response to radiotherapy, a plasticity that could explain their radioresistance and the systematic presence of recurrence, even after surgical resection and radio-chemotherapy. He was able to demonstrate that ionising radiation causes phenotypic and metabolic plasticity in patients’ Glioblastoma cells, as well as the acquisition of cancer stem cell-like traits and functions, a phenomenon known as dedifferentiation (Dahan et al 2014 PMID: 25429620). The research of this group has focused on the identification of new radiosensitising targets specific to these Glioblastoma stem cells by identifying in particular integrin b8 (Malric et al 2017 & 2019, PMID: 29156849 and PMID: 30266751) and survivin (Dahan et al 2014 PMID: 25429620).
The line of research developed today focuses on the study of intra-patient tumour heterogeneity and its role in radioresistance (Boyrie et al 2018, PMID: 30333910). It has been shown by the team that metabolically active areas of the tumour defined by MRI spectroscopy in gliobalstoma patients (Laprie et al, 2008 PMID: 18262090; Deviers et al 2014 PMID: 25104068), known to be specific sites of tumour recurrence, were enriched in Glioblastoma stem cells (Lemarié et al in preparation). This project therefore focuses on this intra-tumoral heterogeneity in order to characterise the cancer stem cells from these areas and to establish their radioresistance profile, their transcriptomic profile (RNAseq), and their metabolic profile (metabolome analysis, Seahorse flow analyser), both in vitro and in vivo (MRI spectroscopy method). The objective of this project is to identify specific metabolic targets for these Glioblastoma stem cells in order to better radiosensitize these metabolically active areas in the patient and thus block tumor recurrence.
Key words
Metabolism, plasticity, dedifferentiation, Glioblastoma stem cells, tumour heterogeneity
Partners and funders
Ligue contre le Cancer (Tarn, Hautes-Pyrénées), ARTC, FRM, INCA Plan Cancer, canceropôle GSO
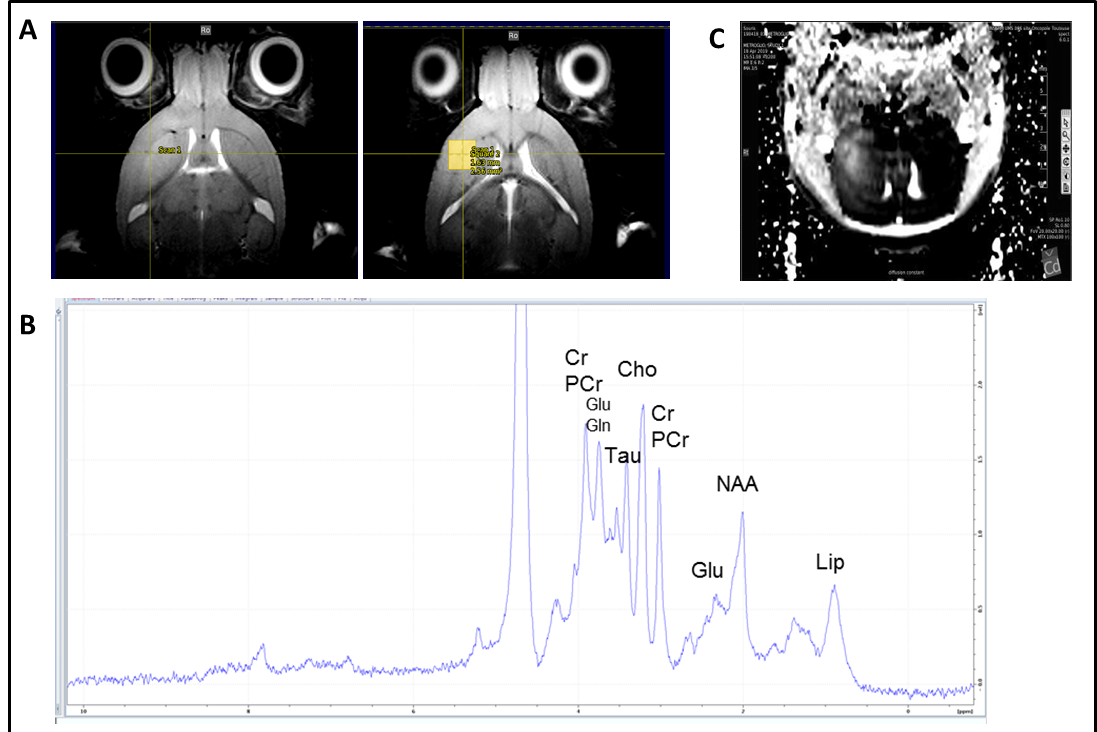
Spectroscopie IRM et IRM multimodale chez la souris xénogreffée en orthotopie par des cellules souches de glioblastome (GB) générée à partir de patients atteinds de GB. Après la croissance de la tumeur, le cerveau de la souris a été soumis à l’IRM multimodale et à la spectroscopie IRM. Séquences d’imagerie IRM montrant une zone tumorale d’hyperintensité et délimitation de la zone (voxel) soumise à la spectroscopie IRM (à droite). (B) Spectre de spectroscopie IRM du voxel tumoral défini en (A) avec les pics des différents métabolites mis en évidence. (C) Séquence d’imagerie IRM de diffusion montrant une zone d’hyperdiffusion dans la région tumorale. Collaboration avec F. Desmoulin, plateforme d’imagerie du petit animal, UMS006 CREFRE , Toulouse / MRI and multimodal MRI spectroscopy in mice orthotopically xenografted with glioblastoma (GB) stem cells generated from GB patients. After tumor growth, the mouse brain was subjected to multimodal MRI and MRI spectroscopy. MRI imaging sequences showing a tumor area of hyperintensity and delineation of the area (voxel) subjected to MRI spectroscopy (right). (B) MRI spectroscopy spectrum of the tumor voxel defined in (A) with the peaks of the different metabolites highlighted. (C) Diffusion MRI imaging sequence showing an area of hyperdiffusion in the tumor region. Collaboration with F. Desmoulin, small animal imaging platform, UMS006 CREFRE, Toulouse

