Preclinical development of non-viral gene therapy for patients with advanced pancreatic cancer
Pancreatic cancer,
non-viral gene therapy,
resistance to treatment,
chemotherapy,
preclinical regulatory experiments.
Pierre Cordelier Team ImPact– Therapeutic innovation in pancreatic cancer.
Pancreatic cancer remains one of the greatest challenges in oncology for which therapeutic intervention is urgently needed. We previously demonstrated that the intratumoral gene transfer of somatostatin receptor 2, to combat tumor aggressiveness, or of deoxycytidine kinase and uridylate monophosphate kinase, to sensitize to gemcitabine chemotherapy, has antitumoral potential in experimental models of cancer. Here, we describe the development of the CYL-02 non-viral gene-therapy product, that comprises a DNA-plasmid encoding for the three aforementioned genes which expression is targeted to tumor cells, and complexed with PolyEthylEnimine non-viral vector. We performed preclinical toxicology, biodistribution and therapeutic activity studies of CYL-02 in two rodent models of pancreatic cancer. We found that CYL-02 is safe, does not increase gemcitabine toxicity, is rapidly cleared from blood following intravenous administration, and sequestered in tumors following intratumoral injection. CYL-02 drives the expression of therapeutic genes in cancer cells and strongly sensitizes tumor cells to gemcitabine, both in vitro and in vivo, with significant inhibition of tumor cells dissemination. This study was instrumental for the later use of CYL-02 in patients with advanced pancreatic cancer, demonstrating that rigorous and thorough preclinical investigations are informative for the clinical transfer of gene therapies against this disease.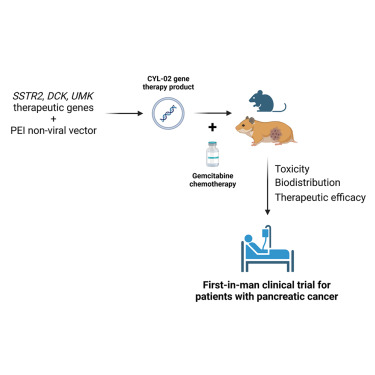
Discover the published article
Mol Ther Methods Clin Dev , Mars 2023, doi:https://doi.org/10.1016/j.omtm.2023.03.005
Preclinical development of non-viral gene therapy for patients with advanced pancreatic cancer
Barbey O, Lulka H, Hanoun N, Belhadj-Tahar H, Vernejoul F, Cambois G,Tiraby M, Buscail L, Gross F, Cordelier P,
Collaborations and acknowledgements
Scientific partners:
- Toulouse University Hospital,
- Invivogen company and its founder Mr. Gérard Tiraby to whom this article is dedicated
Funders:
- ANR,
- Inserm,
- Occitanie Region

Toulouse Cancer Research Center (Oncopole)
Toulouse - FR
Contact us
+33 5 82 74 15 75
Want to join
the CRCT team ?




