
Ludovic MARTINET
Mechanisms driving tumor associated cytotoxic T cell dysfunction
Unlocking the brakes of cytotoxic lymphocytes through PD1 and CTLA-4 immune checkpoint blockade represents the most efficient anti-cancer treatment in metastatic disease. Unfortunately, it becomes evident that such approaches only benefit to a limited number of cancer type and patients. Therefore, finding additional molecules that limit anti-tumor immune responses represents a major public health issue.
Dysregulated inflammation, a Key Driver of Myeloma Immunosuppression.
The growth of myeloma cells highly depends on the bone marrow, where cellular components and soluble factors cooperatively orchestrate a pro-survival and immunosuppressive microenvironment. However, the molecular and cellular mechanisms of myeloma-associated inflammation and immunosuppression remained poorly understood. Using Vk*MYC preclinical mouse model, a comprehensive analysis of the transcriptional landscape of the immune microenvironment in myeloma patients, we recently demonstrated, in collaboration with Pr Smyth Laboratory (QIMR Berghofer), that dysregulated production of IL-18 is a key driving force for immunosuppression in the myeloma microenvironment and a potential therapeutic target (Nakamura, Kassem et al. 2018).Tumor-promoting inflammation and avoiding immune destruction were already known as cancer driving hallmarks. Our results demonstrated that the pro-inflammatory cytokines are critically involved in these hallmarks in multiple myeloma providing new insights into therapeutic strategies against this disease. A comprehensive analysis of inflammatory network that may alter the efficacy of immune effector cells is now required.
Eomes-dependent CD226 Loss restrains CD8+ T Lymphocyte Anti-tumor functions.
The mechanisms underlying the lack of responsiveness of anti-tumor CD8+ T cells are still poorly understood and finding additional signals that regulate their functions has become a major priority. In a recent study, we discovered that the loss of the activating receptor CD226 restrains CD8+ T cell functions and the therapeutic efficacy of cancer immunotherapy (Weulersse et al, immunity 2020). The aim of our project is now to use cancer patients’ samples as well as the most relevant tumor mouse models to further dissect the molecular role of key activating receptors in CD8+ TCR signaling, in anti-tumor immune responses and in the efficacy of monoclonal antibody-based cancer immunotherapy.
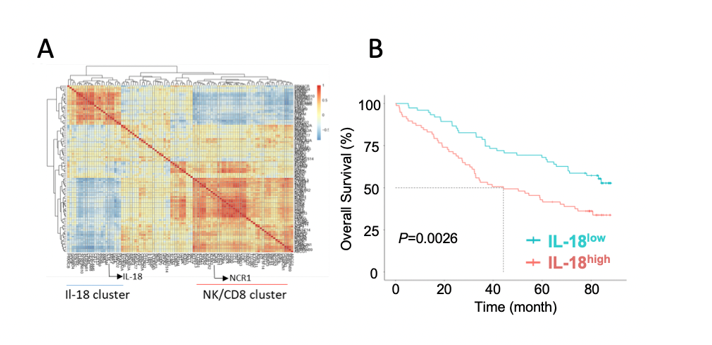
Figure 1: Chronic inflammation as potential source of cytotoxic cell dysfunctions in MM. A. Heatmap showing the inverse correlation between IL-18 and NK/CD8 gene clusters within CD138– BM aspirates from 73 MM patients at diagnosis analyzed by RNA sequencing. B. Kaplan–Meier survival estimates over more than 80 months of follow-up for IL-18high (> median value) and IL-18low (< median value) MM patients.
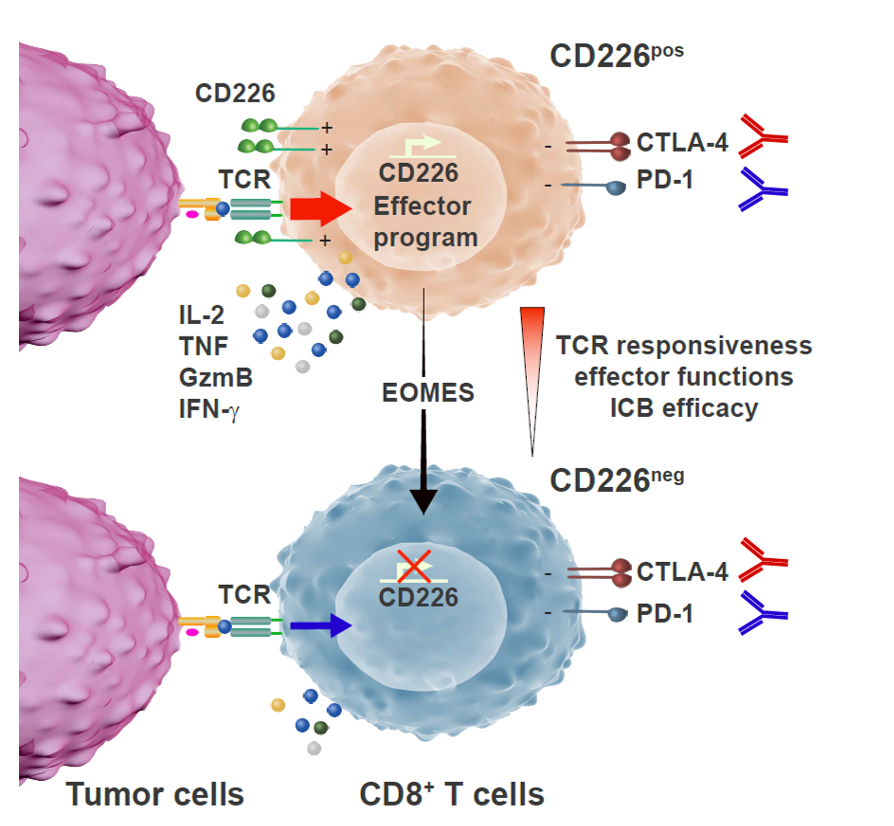
Figure 2: the loss of the activating receptor CD226 restrains CD8+ T cell functions and the therapeutic efficacy of cancer immunotherapy.

