Quantifying the sources of inter-individual variability in hormone therapy exposure.
Dr Fabienne Thomas, Dr Mélanie White-Koning et Dr Cécile Arellano
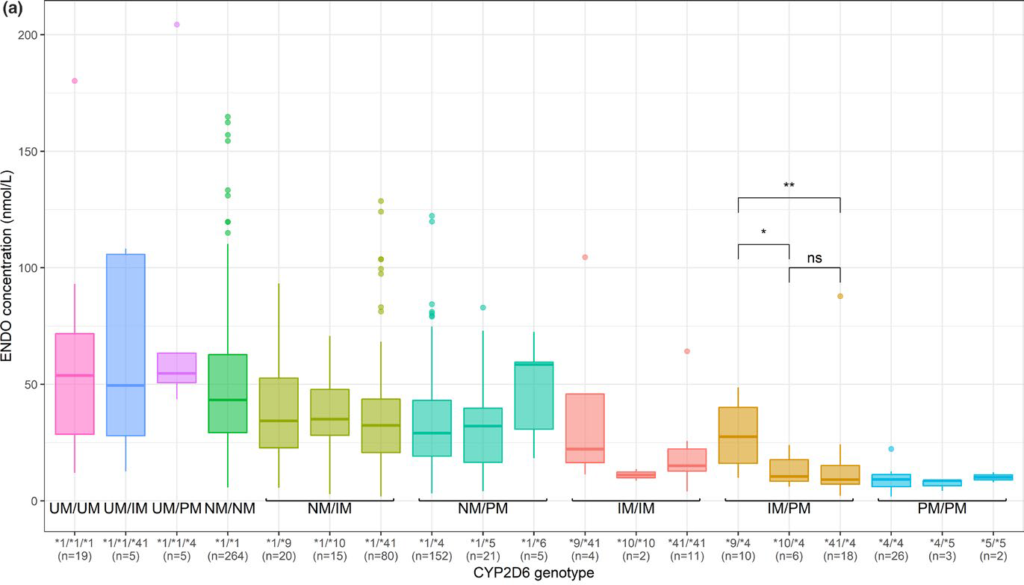
The PHACS project, in which 2000 patients treated with adjuvant hormone therapy (tamoxifen, letrozole, anastrozole and exemestane) for breast cancer were followed for 3 years, made it possible to determine the plasma concentrations of the molecules under study and to quantify the impact of genetic variants and concomitant treatments on these concentrations. For tamoxifen, we confirmed the important influence of CYP2D6 metaboliser status but also of CYP3A4 and concomitant treatments. The data obtained were used to build a pharmacokinetic model that can be used as a tool for dose adaptation. Ongoing analyses aim to determine the impact of exposure and genetic polymorphisms on the occurrence of adverse effects of tamoxifen and anti-aromatases (letrozole, anastrozole, exemestane).
Financing :
PHRC
Collaborations :
Health establishments (CLCC, CHU, CH and private clinics) in the Greater South-West
Illustration :