
CORINNE BOUSQUET
Identification of tumor-stroma dialogues involved in the aggressiveness of pancreatic cancer
Our recent work has shown the involvement of mRNA translation deregulation in the acquisition of an aggressive phenotype in both pancreatic cancer cells and CAFs (Müller, JCI Insight 2019; Duluc, EMBO Mol Med 2015). In CAFs, this excess of mRNA translation leads to exacerbated secretion of proteins destined for secretion and with pro-tumor activity, the identity of which has been clarified by CAFs secretome analyses (by mass spectrometry). To target this specificity in CAFs, we identified a receptor specifically expressed in CAFs and not in healthy pancreatic fibroblasts, and whose targeting with a drug used in oncology for the treatment of neuroendocrine tumors (Novartis collaboration), allowed to inhibit this excess of protein secretion and thus the pro-metastatic and chemoprotective potential of CAFs (Samain, CMGH 2021). Blocking a protein identified to be secreted by CAFs and shown to be involved in inhibiting cytotoxic CD8 T cell-dependent anti-tumor immunity also slowed tumor progression (Goehrig, Gut 2019).
We have also shown that several CAF subpopulations co-exist in the same tumor (Neuzillet, J Pathol 2019), with potentially different functions and plasticity evolving with tumor progression and/or response to treatments. Our current projects therefore consist in searching for specific vulnerabilities of fibroblast populations present in the most aggressive tumors, by analyzing their transcriptome and translatome (PDX models, patient-derived xenograft) in relation to the clinic (anatomo-pathological and clinical data of patients). We carry out this project both in treatment-naive tumors (patients operated before being treated with chemotherapy), and after chemotherapy, in order to identify the mechanisms of post-treatment relapse, with the hypothesis that the microenvironment, and more specifically CAFs, are strongly involved.
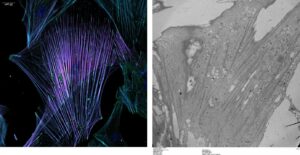
Cancer-associated fibroblast (left: immunofluorescence allowing visualization of the actin cytoskeleton in purple; right: transmission electron microscopy).
Funding: Labellisation Ligue Nationale Contre le Cancer, INCa (PAIR Pancreas and PlBIO), Plan Cancer 3R, ARC, FRM, PHC France-Belgium.
Members: Marjorie Fanjul (MCU, Toulouse 3), Emilie Decaup (Research Engineer, Cancer Plan), Hippolyte Audureau (Study Engineer, INCa), Alexia Brunel (Thesis, LNCC / FRM).
Recent references:
- Samain R, et al. Pharmacological normalization of pancreatic cancer-associated fibroblast secretome impairs pro-metastatic cross-talk with macrophages. Cell Mol Gastro Hepatol. 2021. PMID: 33482394.
- Müller D, et al. (2019) eIF4A inhibition circumvents uncontrolled DNA replication mediated by 4E-BP1 loss in pancreatic cancer. JCI 2019. PMID: 31672935.
- Goehrig D, et al. Stromal protein βig-h3 reprogrammes tumour microenvironment in pancreatic cancer. 2019. PMID: 30415234.
- Neuzillet C, et al. Inter- and intra-tumoral heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J Pathol. PMID: 30575030.
- Duluc C, et al. (2015) Pharmacological targeting of the protein synthesis mTOR/4E-BP1 pathway in cancer-associated fibroblasts abrogates pancreatic tumour chemoresistance. EMBO Mol Med. PMID: 25834145.

