Exploring transcription in oncogenesis and response to anticancer treatment
Agnese Cristini
our research area
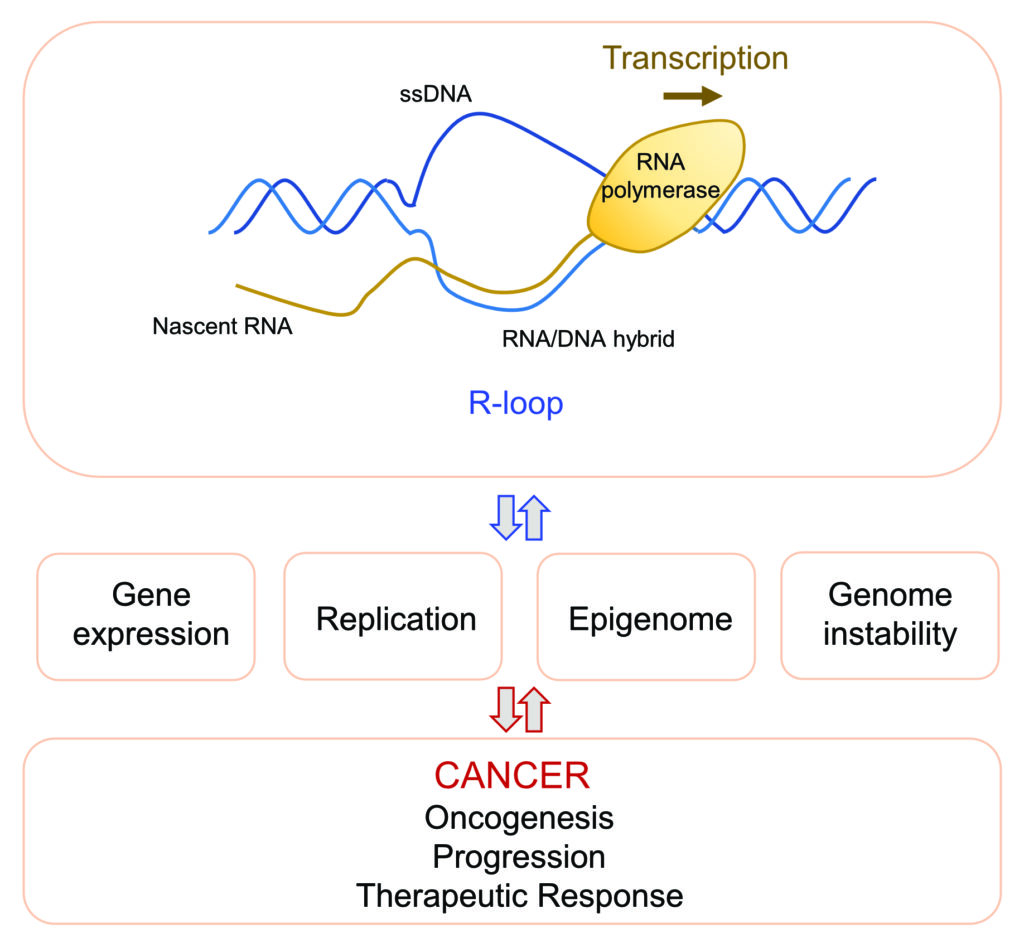
Genetic and epigenetic alterations trigger deregulated gene expression, which is a hallmark of cancer. Cancer cells are “transcriptionally addicted” to these dysregulated transcriptional programs which help sustaining tumour growth and survival. Transcription is associated with the formation of nucleic acid structures, called R-loops, which consist of an RNA/DNA hybrid and a displaced single-stranded DNA. These structures display a complex interplay with key cellular processes including gene expression, replication and maintenance of genomic and epigenomic stability, which are all involved in cancer.
Our research focuses on investigating the molecular mechanisms by which transcription contributes to cancer onset, evolution and response to anticancer therapies. For this, we employ a combination of genome-wide, high-content, biochemical, cellular and molecular approaches.
If you are interested in joining us, please contact us by email (agnese.cristini@inserm.fr) to get more information.
Keywords :
- Cancer
- Transcription
- R-loop
- Gene Expression
- Genome Instability
- DNA Damage
- Oncogenesis
- Anticancer Therapy
- Targeted Therapy
- Chromatin
- Epigenome
Group members :
- Lara Fernandez Martinez, PhD student
- Elisona Shyti, Erasmus and Master 2 student
- Eglantine Faget, Master 1 student
Other team members involved
- Olivier Sordet, PhD, HDR, Research Scientist (CRCN)
- Olivier Calvayrac, PhD, Research Scientist (CRCN)
- Anne Pradines, PhD, HDR, Researcher
- Mathéa Géraud, PhD student
- Andrea Carla Ajello, PhD student
- Anne Casanova, Assistant Engineer
Selected publications :
Nat Commun. 2022 May 26;13(1):2961. doi: 10.1038/s41467-022-30604-0. PMID: 35618715
Cristini A, Tellier M, Constantinescu F, Accalai C, Albulescu LO, Heiringhoff R, Bery N, Sordet O, Murphy S, and Gromak N.
RNase H2, mutated in Aicardi-Goutieres syndrome, resolves co-transcriptional R-loops to prevent DNA breaks and inflammation.
Int Rev Cell Mol Biol. 2021 Sep 12;364:195-240. doi: 10.1016/bs.ircmb.2021.05.001. PMID: 34507784.
Cristini A*, Géraud M, Sordet O*.
Transcription-associated DNA breaks and cancer: A matter of DNA topology.
*Co-corresponding authors.
Mol Cell Oncol. 2020 Jan 10;7(2):1691905. doi: 10.1080/23723556.2019.1691905. eCollection 2020. PMID: 32158914
Cristini A, Gromak N, Sordet O.
Transcription-dependent DNA double-strand breaks and human disease.
Cell Rep. 2019 Sep 17;28(12):3167-3181.e6. doi: 10.1016/j.celrep.2019.08.041. PMID: 31533039
Cristini A, Ricci G, Britton S, Salimbeni S, Huang SN, Marinello J, Calsou P, Pommier Y, Favre G, Capranico G, Gromak N, Sordet O.
Dual Processing of R-Loops and Topoisomerase I Induces Transcription-Dependent DNA Double-Strand Breaks.
Cell Rep. 2018 May 8;23(6):1891-1905. doi: 10.1016/j.celrep.2018.04.025. PMID: 29742442
Cristini A*, Groh M*, Kristiansen MS, Gromak N.
RNA/DNA Hybrid Interactome Identifies DXH9 as a Molecular Player in Transcriptional Termination and R-Loop-Associated DNA Damage.
*Co-first authors.