Setting up clinical trials and immune monitoring of patients treated with immunotherapy.
Clara-Maria Scarlata
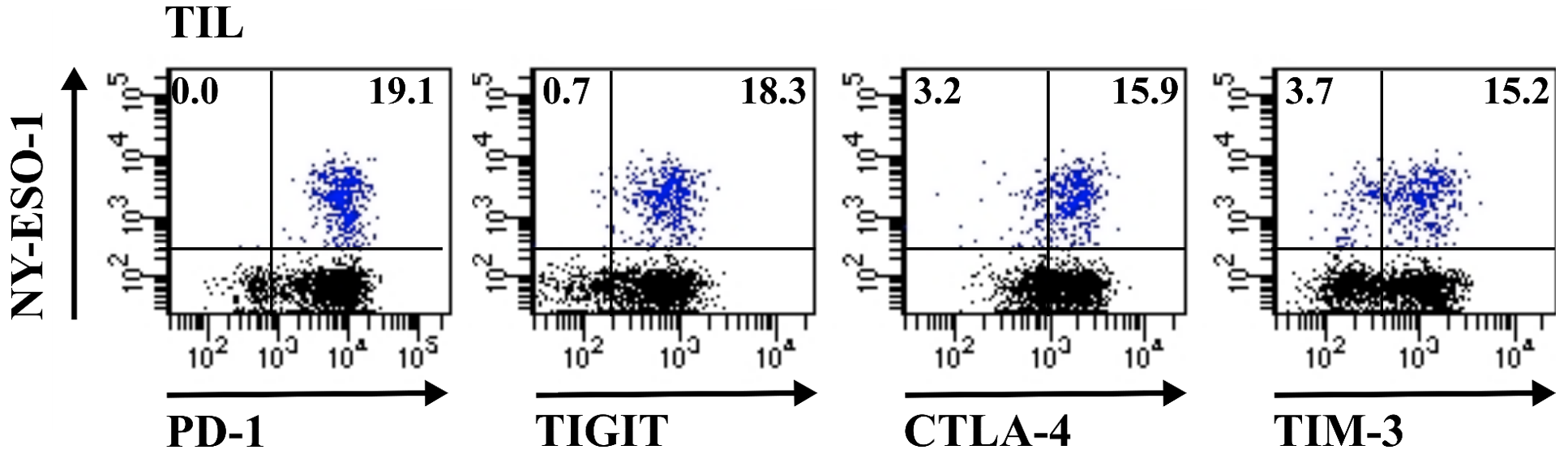
All our results and the scientific literature converge on the need to induce or increase the anti-tumour T immune response in order to improve the clinical response to immune checkpoint inhibitors (IPCI). We have participated in the implementation of several clinical trials combining or not these strategies with IPCIs and we ensure, in connection with the promoters of these trials (IUCT-O and pharmaceutical industries) and thanks to our Immunomonitoring Unit, the immunological follow-up of the patients included.
We have chosen two strategies for the induction of T responses:
- Anti-cancer vaccines: the personalised vaccination trial against neoantigens, TG4050 (NCT04183166), developed by Transgene, is emblematic of this axis.
- The use of so-called immunogenic radiotherapy regimens, which we are developing in collaboration with the radiotherapy department of the IUCT-O (Pr. E. Moyal). Ex. SterImGli trial (NCT02866747)
Technologies used
Flow cytometry (17 colour staining); HLA/peptide multimer analysis of antigen-specific T cells; Functional analysis of T cells.